Over three decades of research have gone into developing miniature biosensors capable of identifying single molecules. These devices could be used to detect molecular markers of cancer or other diseases and to evaluate the effectiveness and safety of drugs to combat them.
Scientists must discover ways to understand the interactions of molecules and sensors to make this possible and improve speed and accuracy. Researchers have developed a new approach from Virginia Commonwealth University (VCU) and the National Institute of Standards and Technology. Their findings were published in Science Advances.
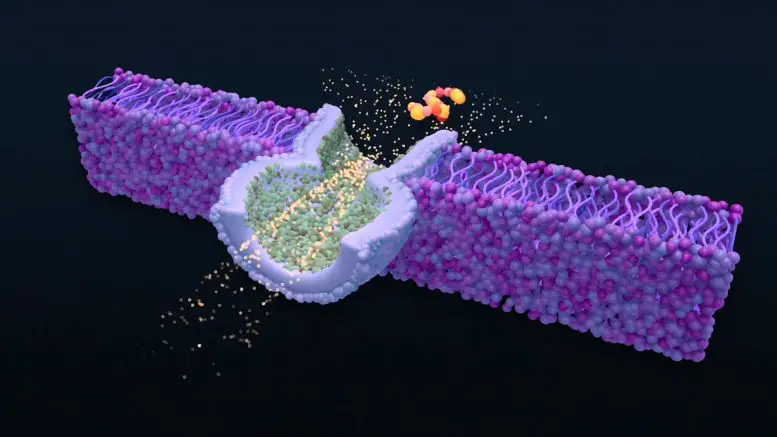
The team built the biosensor using a synthetic version (biomaterial) of the biological material that forms cell membranes. It is also known as a bilayer of lipids. It has a tiny pore that measures 2 nanometers in size. The pore is surrounded by fluid. The nanopore allows ions dissolved in liquid to pass through, creating a small electric current. The membrane partially blocks current flow when a molecule of particular interest is driven into it. This blockade’s duration and strength can be used as a fingerprint to identify the properties and size of a specific molecule.
For accurate measurements of large molecules, they must remain in the nanopore for a short and long time (the “Goldilocks”), ranging from 100 millionths to 10 thousandths of seconds. Most molecules will remain in the nanopore’s small volume for this period if the nanopore holds them in place. The nanopore environment must create a barrier that allows molecules to escape, such as an electrostatic force or change in its shape.
Each molecule type has a different minimum energy required to break the barrier. This is crucial for the biosensor’s ability to function efficiently and accurately. This quantity can be calculated by measuring many properties of molecules as they move into and out of the pore.
It is crucial to determine whether the interaction between the molecule of its environment and the captured and released molecules arises primarily out of a chemical bond or from the ability to move and wiggle freely during the capture and release process.
For technical reasons, obtaining reliable measurements of these energetic components has been challenging. A team led by Joseph Robertson from NIST and Joseph Reiner from VCU showed they could measure these energies using a laser-based heating method.
Measurements must be done at various temperatures. The laser heating system ensures that temperature changes are rapid and reproducible. Researchers can complete measures in less time than 30 minutes.
Robertson stated, “Without the new laser-based heating device, our experience suggests they won’t be done; it would be too expensive and time-consuming to do them.” He said, “We have created a tool to change the development pipeline of nanopore sensors to reduce the guesswork involved with sensor discovery quickly.”
After energy measurements have been taken, it is possible to determine how a molecule interacts and interacts with the nanopore. Scientists can use this information to identify the most effective molecule detection strategies.
Consider a molecule that interacts with the nanopore through chemical, essentially electrostatic interactions. The researchers tried to modify the nanopore’s electrostatic attraction to the target molecules to be strong enough to achieve Goldilocks capture.
The researchers developed the method using two small peptides. These short chains of compounds make up the building blocks for proteins. Angiotensin, one of the peptides that stabilize blood pressure, is another. Neurotensin is the other peptide that regulates dopamine. This neurotransmitter influences mood and could also play a part in colorectal carcinoma. These molecules interact with nanopores primarily via electrostatic forces. Researchers inserted nanopore-sized gold nanoparticles capped with a charge material to increase their electrostatic interactions.
The team, polyethylene glycol, also studied another molecule. Its ability to move determines how long it spends inside the nanopore. This molecule can move freely and rotate, wiggle, and stretch without being restricted by its environment. Researchers altered the shape of the nanopore to increase the molecular’s stay in the nanopore. This made it harder for the molecule, which could not escape through the tiny hole and escape.
Robertson says, “We can use these changes to create a nanopore-biosensor tailored for detecting specific molecules.” A research lab could use a biosensor to identify biological molecules of particular interest or a doctor’s practice to identify disease markers.
Robertson stated, “Our measurements give us a blueprint for how to modify the interactions in the pore, whether through geometry or chemical or a combination of both, and tailor a nanopore sensor to detect specific molecules, count small numbers of molecules, or both.”